Osteonecrosis
<-Osteopenia | Skeletal Dysplasias->
General
Osteonecrosis bespeaks bone death. Synonyms include aseptic necrosis, bone necrosis, avascular necrosis, bone infarction and ischemic necrosis. By convention, the terms “aseptic” or “avascular” necrosis have been applied to areas of juxtaarticular involvement and the term bone infarct is usually applied to metaphyseal or diaphyseal involvement. However, this terminology is not applied consistently throughout the literature, and I feel that these are largely artificial distinctions. I prefer to simply use the single term ‘osteonecrosis’ and to apply it to areas of bone infarction, no matter where in the bone they occur.
Mechanisms of Osteonecrosis
There are many paths to satori, and likewise to osteonecrosis. Osteonecrosis is multifactorial in etiology, and has a widely differing prognosis, depending upon the exact part of the bone involved by the necrosis. The exact mechanism of osteonecrosis depends primarily on the predisposing cause. This is reflected in the table below.
Mnemonic = VINDICATE
Differential Diagnosis of Osteonecrosis
| Category of Disease | Disorder | Mechanism |
| Vascular | sickle cell disease and other hemoglobinopathies, polycythemia vera and other lymphoproliferative disorders |
sludging of cells in vessels |
| pregnancy | impaired venous drainage caused by the gravid uterus | |
| Infection | septic emboli | causing arterial occlusion |
| Drugs / Toxins | steroid use | fat cell enlargement causing medullary hypertension and intraosseous venous occlusion |
| alcoholism | fatty emboli causing arterial occlusion | |
| Inflammatory | pancreatitis | fatty emboli causing arterial occlusion |
| Congenital | Gaucher’s disease | marrow packing by Gaucher cells causing medullary hypertension and intraosseous venous occlusion |
| Autoimmune | Systemic lupus erythematosus, rheumatoid arthritis | vasculitis causing arterial occlusion |
| Trauma | fracture / dislocation | interruption of arterial supply to bone (e.g. scaphoid fracture, hip dislocation, talar neck fracture) |
| radiation | direct injury to vascular supply leading to occlusion | |
| dysbarism (caisson disease) | nitrogen emboli causing arterial occlusion / accumulation of nitrogen bubbles in marrow fat |
|
| thermal trauma (burns, frostbite) | direct tissue damage | |
| Endocrine / Metabolic | Cushing’s disease | fat cell enlargement causing medullary hypertension and intraosseous venous occlusion |
It seems logical that the sequence of events in developing osteonecrosis should be as follows:
- some phenomenon causing decreased blood flow to bone
- bone ischemia, if the flow becomes low enough
- bone infarction, if the flow becomes lower still
Osteonecrosis is generally thought of being an irreversible process. If the necrosis occurs next to a joint surface, it is generally considered to cause joint deformity. However, no one really knows if this is true, and such conclusions are to some extent artifacts of our current diagnostic methods for osteonecrosis. With the advent of MRI, we finally have a tool which can show abnormalities within about 2 weeks of the original insult. However, current standard MR scanning cannot yet show abnormalities at the instant of infarction. What is needed to answer this question is a diagnostic technique that is sensitive to vascular flow, and not just intensity changes in the bone marrow. Dynamic MR scanning following a gadolinium bolus may be helpful in this regard, but definitive studies have not yet been done to prove this speculation.
There is some evidence in the literature that suggests that in some circumstances, osteonecrosis may be a reversible process that can resolve completely without subchondral collapse and subsequent joint arthrosis. This evidence is found in the literature for transient osteoporosis of the hip, an idiopathic disorder characterized by a reversible osteopenia of the hip and marked marrow edema. If this definition sounds a bit circular, well, that’s how idiopathic disorders are, isn’t it. We don’t know what causes them, so their names pretty well sum up most of what we know about them. Anyhow, several studies of TOH have been published in which the characteristic edematous marrow of TOH was biopsied. These biopsies have shown the presence of osteonecrosis in many cases, leading to the suggestion that TOH may actually be due to a low-grade and reversible osteonecrosis.
Concave / convex joint physiology
It turns out that, in part, the shape of joints governs the distribution of osteonecrosis. In particular, it is the convex versus concave shape of the opposing joint surfaces of most joints that determines this distribution (Simkin et al).
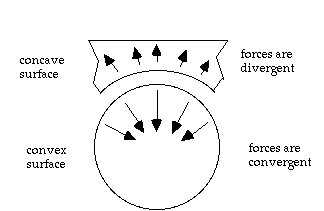
On both sides of the joint, the forces of weight-bearing and other loading are directed tangential to the joint surface. On the convex side of the joint, these forces converge to a common center. On the concave side, these forces diverge away from each other. To see the significance of this fact, one must first consider the phenomenon of hydraulic resistance. The human body, including the bones are composed of about 50 – 67% water by mass. One can think of the bones as calcium balloons filled with water. Water is not compressible, so this internal water provides some support to the bones, and this supporting force is called hydraulic resistance. Nature is thrifty, and the presence of hydraulic resistance makes it possible to provide the same support with less bone. Since the loading forces converge to a common center on the convex side of the joint, hydraulic resistance becomes a fairly efficient mechanism for resisting these forces. On the concave side of the joint, the divergent nature of the forces makes this mechanism much less efficient, and therefore, hydraulic resistance plays a much smaller role in bone support. The two sides of the bone are obviously supporting the same load, so more bone is therefore necessary on the concave side. This is seen in the form of increased thickness of the subchondral bone along the concave side of the joint in virtually every concave/convex joint pair in the body.
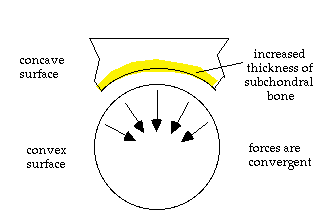
The presence of this extra bone along the subchondral portion of the concave joint surface has some important ramifications for osteonecrosis. Consider first the normal joint and how intramedullary pressure varies on both sides of the joint with loading. Since hydraulic resistance is used as an important support on the convex side of the joint, it is not surprising that the intramedullary pressure on this side of the joint will vary widely with loading. Due to the extra bone supporting the concave side, there is less variation of the intramedullary pressure on that side of the joint, as shown below.
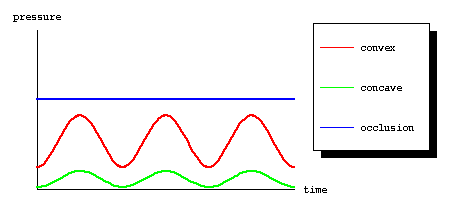
Now, given this picture of normal variations in intramedullary pressure with loading, consider how the patient developing osteonecrosis. Specifically, consider a patient on steroids, probably the most common cause of osteonecrosis. Chronic steroid use causes fat cells to grow. This is the basis for such well-known physical findings such as the “buffalo hump” or the “moon facies” seen in these patients. Many people don’t realize that the intramedullary fat cells share in this growth. As the patient spends more and more time on steroids and the intramedullary fat cells grow larger and larger, the baseline intramedullary pressure begins to rise. Why? Because the bone is a closed and fairly stiff cavity, and will not give with increases in intramedullary pressure. This is an analogous situation to the skull in cases of cerebral edema. However, in this case, the casualty is not the brain, but the intramedullary veins. The intramedullary arteries operate at systemic systolic pressure (90 – 140 mm Hg), but the intramedullary veins operate at a very low pressure (3 – 5 mm Hg). Therefore, the intramedullary pressure doesn’t have to rise very high to cause occlusion of the intramedullary veins. As the intramedullary pressure rises, this leads initially to intermittent occlusion during loading on the convex side of the joint.
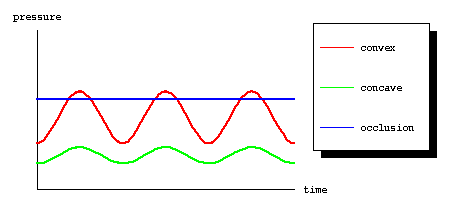
However, as the pressure continues to rise, the intramedullary veins on the convex side of the joint finally become occluded all of the time.
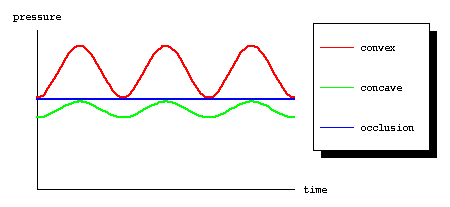
Methods of diagnosisThe mechanism outlined here helps to explain the overwhelming distribution of osteonecrosis toward the convex side of the joint. No matter how much experience you’ve had looking at osteonecrosis, most of the cases of joint involvement that you’ve seen involved the convex side, whether you noticed it or not. In my personal experience with hundreds of cases, I’ve only seen joint involvement on the concave side in two or three cases. Your mileage may vary.
Once osteonecrosis is pretty well established, just about every imaging method will reveal it. Other than MRI, most of these methods are not terribly sensitive, and the osteonecrosis will be well developed by the time that it is diagnosed, as shown in the table below.
| Imaging Method | Findings | Time to Diagnosis | Comments |
| CT | reactive sclerosis subchondral collapse |
months | sensitivity poor specificity OK |
| radionuclide imaging | decreased uptake early, increased uptake late | weeks | sensitivity good specificity poor |
| MRI | decreased signal in a segmental pattern | days to weeks | sensitivity excellent specificity good |
| MRI or PET flow study | decreased flow through affected bone | minutes | theoretically possible, but not yet proven |
Approach to Osteonecrosis
Generally the diagnosis of osteonecrosis is not too difficult. The patient often has some known condition that places them at high risk for osteonecrosis, and the radiographic requisition states something like “R/O AVN”. In such cases, it is usually not difficult to twig to the idea that osteonecrosis belongs in the differential.
Typical plain film findings
The earliest radiographic appearance of osteonecrosis is zilch. That is, the radiograph looks completely normal. After weeks to a month or two, the patient may develop an ill-defined mottling of the trabecular pattern as the earliest evidence of osteonecrosis. Early on, this is so ill-defined that most radiologists will miss it, unless they have a lot of experience looking at osteonecrosis. The late findings of osteonecrosis depend upon its location within the bone. If the lesion occurs in the medullary space well away from the joint, one eventually may see the classic pattern of dense, serpiginous calcification. However, if the necrosis occurs in the subchondral bone, a different pattern usually emerges. Once the osteonecrosis has been present for months, microfractures will accumulate in the dead bone to the point that one may see developing subchondral fractures. This may lead to a discontinuity in the subchondral line, or in some cases, to the “crescent sign”, which represents a fracture between the subchondral line and adjacent necrotic bone. As living bone reacts to the presence of adjacent dead bone, a thick sclerotic zone may develop along the “no-man’s land” between the living and necrotic bone.
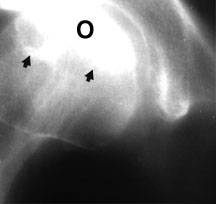
Tomogram of the right hip showing a segmental zone of sclerosis (arrows) in the superior portion of the femoral head, consistent with osteonecrosis (O).
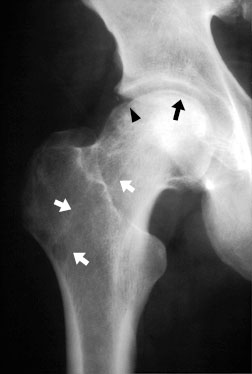
AP radiograph of the hip in a patient with osteonecrosis. Note the lucent crescent sign (black arrow) and the discontinuity where the subchondral bone has collapsed (black arrowhead). Also note a core decompression channel drilled up through the femoral neck (white arrows).
Typical MR findings
The classic MR appearance of osteonecrosis is that of a segmental area of low signal intensity in the subchondral bone, bounded by a low signal intensity border. This border may sometimes appear as a dark line adjacent to a bright line — the so-called “double line sign”. Occasionally osteonecrosis will present as an area of diffuse low signal intensity, which may be difficult to differentiate from other entities, such as osteomyelitis, stress fracture, etc.
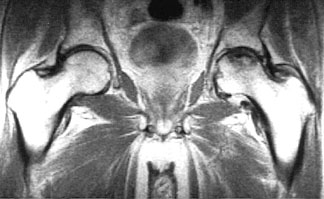
Coronal T1-weighted image of both hips — the low signal intensity in the superior weight-bearing area of the left femoral head is typical for osteonecrosis.
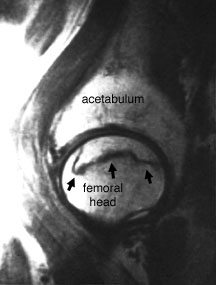
Sagittal T1-weighted surface coil image of the femoral head — this image demonstrates a classic segmental area of osteonecrosis with a dark line denoting the border between dead bone and living bone.
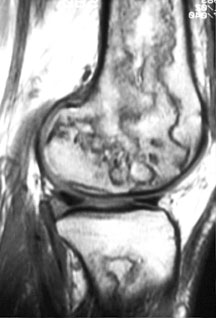
Sagittal T1-weighted image of the knee — this image demonstrates multiple segmental areas of osteonecrosis in the distal femur in this patient with Gaucher’s syndrome.
Fortunately, the plain film and MR findings of osteonecrosis are usually so typical that one does not often have to offer a differential diagnosis. However, sometimes, osteoarthritis, subchondral cysts, transient osteoporosis of the hip and other entities may mimic osteonecrosis on flain films or MR. Sometimes osteonecrosis is unsuspected, and the alert radiologist must consider it whenever unexplained sclerosis or lucency is noted adjacent to a joint, or whenever a patient presents with diffuse skeletal sclerosis.
Besides confirming the diagnosis, another major role for the radiologist in the workup of osteonecrosis is staging the current state of the disease. The usual plain film and MR stages are listed below.
| Stage | Findings |
| 0 | asymptomatic, normal radiographs |
| I | normal radiographs (abnormal MRI) |
| II | radiolucency and sclerosis |
| III | crescent sign, normal contour |
| IV | subchondral collapse, flattening |
| V | degenerative joint disease |
Wise sayings about osteonecrosis
- Remember the concave/convex joint physiology and its effect on the distribution of osteonecrosis about a joint.
- In a patient with diffuse sclerosis, consider some diffuse cause of osteonecrosis, especially sickle cell disease.
References
- Simkin PA, Graney DO, Fiechtner JJ. Roman arches, human joints, and disease: differences between convex and concave sides of joints. Arthritis Rheum 1980;23:1308-1311.