Osteopenia
General
One of the most common findings in skeletal radiology is increased radiolucency of bone, most properly termed osteopenia. This term is much preferred over terms such as “demineralization” or “undermineralization”, since we really can’t tell the exact mineral status of the patient’s bone from the radiograph alone. The most common cause by far of osteopenia is osteoporosis. However, there are many disease entities that can cause osteopenia, so the mere finding of radiolucent bone does not make this an automatic diagnosis. Rather, it prompts a search for other more specific clues to the exact underlying disorder. For example, the table below shows several disorders that can produce osteopenia, as well as more specific radiographic clues to their diagnosis.
| Disorder | Specific radiographic clues |
| osteomalacia | Looser zones |
| hyperparathyroidism | subperiosteal resorption |
| disseminated multiple myeloma | focal lytic lesions |
It can be fairly difficult to diagnose osteopenia accurately on plain radiographs. First of all, plain films are hideously insensitive to changes in bone mineral. One must lose 30 – 50 % of the bone mass before it can be detected on a plain film. Another problem with plain films is the lack of some standard by which to compare the area of interest. Finally, differences in radiographic technique can widely alter the radiologist’s perception of whether or not osteopenia is present. Indeed, Dr. Resnick has made the statement that “You can take a normal patient and an abnormal X-ray technician, and give the patient osteoporosis at will.”
Approach
The approach to osteopenia can be simplified greatly, if one forgets all causes except osteoporosis. Osteoporosis is, by far, the most likely cause of osteopenia. In fact, if you just dropped the term osteopenia and used the term osteoporosis instead, you would be right about 95 % of the time, which is not a bad batting average. This may not be a bad strategy for the larval radiologist, but as you move up the radiologic food chain, you should strive to do better than this. Remember that 95 % diagnostic accuracy is easier to accept if you and your family are not in the other 5 %. Also remember that if all you ever do is blindly apply a bunch of 95 % rules, you can be replaced by a file room clerk with a rubber stamp. In these days of cost containment, this should be a sobering thought.
A good place to start might be with recognition of the usual patterns of osteopenia seen in everyday life. Once one gets used to the seeing these patterns, then deviations from the usual patterns will begin to stand out, and one can suggest to a clinician that further workup may be indicated in these cases. What are these usual patterns? The main two types are generalized osteoporosis and localized (regional) osteoporosis. The differential diagnosis for regional osteoporosis is a fairly short and manageable one. The differential diagnosis for generalized osteoporosis is a bit longer and more complex.
Generalized Osteoporosis
There are many causes of generalized osteoporosis. Some of the major ones are listed in the table below.
Mnemonic = VINDICATE
Differential Diagnosis of Osteopenia
- Vascular
- anemic states
- Drugs / Dietary Deficiency
- Drugs
- Steroids
- Heparin
- Dietary Deficiency
- Scurvy
- Malnutrition
- Calcium deficiency
- Drugs
- Idiopathic osteoporosis
- Congenital
- Osteogenesis imperfecta
- Toxic
- Alcoholism
- Chronic liver disease
- Endocrine/Metabolic
- senile
- postmenopausal
- pregnancy
- diabetes mellitus
- hyperparathyroidism
- Cushing’s disease
- acromegaly
- hypogonadism
This VINDICATE differential diagnosis may be helpful for recalling unusual causes of osteoporosis, but it is not as useful as it could be for the radiologist’s everyday life. The reason for this is that the entities listed above are not in the order that one is most likely to see them. The most common causes are senile and postmenopausal osteoporosis. The next most common cause is probably drugs (steroids) or dietary deficiency (calcium). After that, I personally lump everything else into one big category and consult the VINDICATE differential. Let’s look as some of these causes in more detail.
Senile and postmenopausal osteoporosis
These two entities are the most common causes of generalized osteoporosis. Senile osteoporosis simply refers to the gradual loss of skeletal mass that is seen with advancing age. Postmenopausal osteoporosis refers to the increased bone loss seen in women following menopause. Both of these processes are very common, and both commonly occur in the same individuals, so the two entities are often lumped together. The pathogenesis of both of these states is not clear, but probably involves a combination of decreased bone production and increased resorption.
In general, the gradual loss of skeletal mass begins in women in the fourth decade and in the fifth or sixth decade of life for men. This bone loss accelerates in women following the menopause, as shown below.
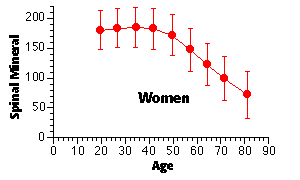
Women lose about 0.75 – 1.0 % of their skeletal mass per year after age 35 — this may accelerate to as much as 2 – 3 % per year following the menopause
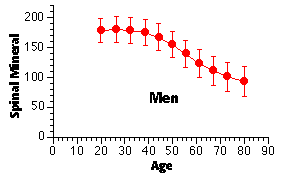
Men lose about 0.4 % of their skeletal mass per year after age 50
Where is this bone lost? It occurs in both cortical and trabecular bone. In the cortex, bone may be lost along the endosteal or subperiosteal surfaces, or may be lost along the surface of the Haversian canals (cortical tunnelling). Trabecular bone loss occurs preferentially among the trabeculae that get the least stress. Conversely, the trabeculae that are spared the longest are those that receive the most stress, generally the primary, vertical weight-bearing trabeculae. In the femur, one sees gradual loss of both the compressive and tensile groups of trabeculae, and the compressive group are usually the last to go. Similar patterns of trabecular bone loss are noted in the spine.
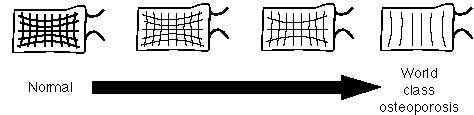
Patterns of trabecular bone loss in the spine
With loss of skeletal mass, one also loses bone strength. This loss of bone strength may be asymptomatic for many years, although significant loss may be accompanied by fractures and bone pain. In the spine, these fractures may take several forms, leaving the vertebral bodies wedged anteriorly, symmetrically flattened (vertebra plana), or with biconcave endplates. Significant vertebral compression may lead to loss of height and increased thoracic kyphosis, sometimes referred to as the “dowager’s hump” in elderly females. Fractures may occur in other areas as well, such as the proximal femur and humerus, distal radius, and ribs.
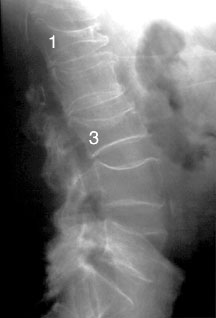
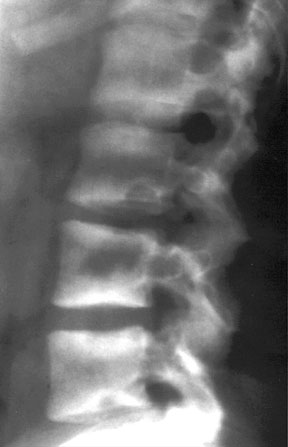
Lateral radiograph of an osteoporotic spine, showing compression fractures in the L1 and L3 vertebral bodies.
It is estimated that approximately 1 million fractures occur annually in the United States in women over 45. Of these, 350,000 could be prevented by the elimination of osteoporosis. This begs the question: just how does one eliminate osteoporosis? Unfortunately, there is not a good answer to this. Despite decades of research, we still don’t really understand why people get osteoporosis with increasing age. We are finally starting to develop treatments for replacing lost skeletal mass, and the research goes on. For now, though, a suitable blanket prescription for most people would consist of a simple regimen of avoiding certain known risk factors for osteoporosis:
- stay active and get plenty of exercise
- eat plenty of supplemental dietary calcium, protein & vitamin C
- don’t smoke
- don’t drink excessively
- strongly consider taking estrogen supplements following menopause
Many techniques have been developed for estimating skeletal mass. These remain primarily a research tool, and their use cannot be recommended for screening the general population. However, they are invaluable in research protocols for testing new treatments for osteoporosis. Most of these techniques rely on some form of calibrated absorptiometry. That is, a known amount of some form of radiation is aimed through a bone, and the amount making it through the bone is measured. The more skeletal mass, the less radiation makes it through to the detector, and vice versa. The archetypal system is the Norland-Cameron single-photon absorptiometer, which was used primarily in the distal radius and ulna, as shown below.
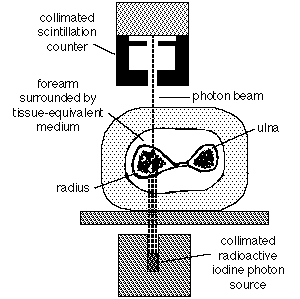
Original Norland-Cameron single photon absorptiometry technique
This original technique has undergone a number of improvements over the years, and has been applied to computed tomography as well. The current standard of choice for skeletal densitometry is known as dual energy X-ray absorptiometry (DEXA), and can be used for virtually any body part desired.
Drugs or deficiency caused osteoporosis
The osteoporosis occurring during Cushing’s syndrome or following exogenous steroid administration is well known. Histologic studies of this process reveal a combination of decreased bone production as well as increased bone resorption. Inadequate dietary calcium intake may lead to osteoporosis. Patients receiving large doses (> 15,000 units/day) of heparin may develop a reversible osteoporosis. Alcoholic patients may also develop reduced bone mass and increased bone fragility, for reasons that are not well understood.
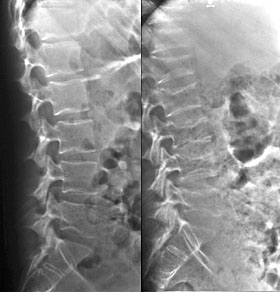
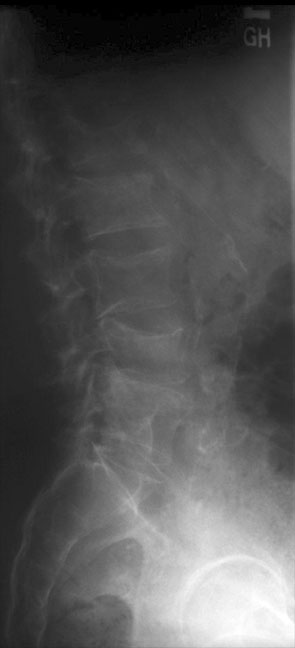
Two views of the lumbar spine taken 1 year apart demonstrate rapidly developing osteoporosis and multiple compression fractures in this patient on exogenous steroids
Other causes of osteoporosis
Hyperthyroidism, acromegaly, pregnancy, idiopathic juvenile osteoporosis and osteogenesis imperfecta are other entities that present with osteoporosis during their course. These are fairly rare causes of osteoporosis, but should be kept in mind when one is faced with unexplained osteoporosis, particularly in younger patients.
Regional osteoporosis
There are several causes of regional osteoporosis. These are shown in the table below.
Major Causes of Regional Osteoporosis
- Immobilization and disuse
- Reflex sympathetic dystrophy syndrome
- Transient regional osteoporosis
- transient regional osteoporosis of the hip
- regional migratory osteoporosis
Disuse osteoporosis
Bone is a dynamically changing organ, and nowhere is this more apparent than in disuse osteoporosis. The mere act of spending a night recumbent in bed is enough to stimulate the process of bone resorption. Increases in calcium excretion rates can be detected easily after a night in bed like this. Once one awakens and returns to a weight-bearing mode, this calcium depletion mode reverses. In prolonged bed rest, however, the amount of bone lost due to this mechanism can be quite severe. This is especially true for astronauts exposed to microgravity environments for lengthy periods. However, this phenomenon is quite commonly seen in the much more mundane circumstances of trauma. Following an extremity injury, the afflicted extremity often spends several weeks in a cast. The lack of normal stresses to the bone can result in a striking osteoporosis distal to and including the area of injury. This is especially evident when compared to the normal bone stock proximal to the area of injury. However, once the injury has healed and normal stresses are restored to the extremity, the bone stock recovers to a large extent, although the period of recovery is several times longer than the period of bone loss, and the degree of recovery shows wide individual variation.
Generally, disuse osteoporosis presents as a diffuse osteopenia seen throughout the disused body part. However, other patterns of osteopenia may also be noted. Lucent bands of osteopenia may be seen just proximal to the physeal line.
Another common pattern is a subchondral distribution to the osteopenia. This subchondral lucency is especially common in the bones of the foot and ankle following trauma. This pattern can even be a helpful prognostic sign in some cases, such as fractures of the talar neck. Fractures in this location frequently disrupt the blood supply to the proximal talar fragment, which may lead to posttraumatic osteonecrosis. However, the presence of subchondral osteopenia in such a fracture is a good prognostic sign, since one cannot develop disuse osteopenia without an intact blood supply to that bone. This is sometimes referred to as the “Hunter-Hawkins sign” or simply “Hawkins sign”.
Reflex sympathetic dystrophy syndrome
Reflex sympathetic dystrophy syndrome (RSDS) is disorder of unclear etiology. It is characterized clinically by pain, vasomotor disturbances (vasospasm or vasodilatation) and trophic skin changes (skin atrophy, pigmentation abnormalities, hypertrichosis, hyperhidrosis and nail changes) and radiographically by regional osteoporosis in the affected area. RSDS was first described in 1864. Since then, it has collected a variety of synonyms, including causalgia, acute bony atrophy, Sudeck’s atrophy or osteodystrophy, posttraumatic osteoporosis, traumatic angiospasm or vasospasm, algodystrophy, reflex dystrophy of the extremities, minor causalgia, postinfarctional sclerodactyly, shoulder-hand syndrome, and reflex neurovascular dystrophy. Currently, the preferred term is RSDS.
The pathogenesis of RSDS remains unclear. A variety of neurally related visceral, musculoskeletal, neurologic, or vascular conditions may be followed by RSDS. In many cases, though, an incipient cause is not identifiable. Reported instigating causes of RSDS include myocardial infarction, cerebrovascular disorders, degenerative disease of the cervical spine, discal herniation, polymyalgia rheumatica, calcific tendinitis, vasculitis, neoplasm elsewhere (lung, brain, breast, ovary, pancreas, bladder, etc.), and a variety of posttraumatic, postsurgical and postinfectious states. The smart money is on the following theory of RSDS: following some lesion or injury, painful impulses travel to the spinal cord, where a series of reflexes are initiated in an “internuncial pool” of neurons. These reflexes then provoke the efferent pathways to the peripheral nerves, where the local findings of RSDS then result.
The diagnosis of RSDS relies primarily on the recognition of the classical clinical findings. Imaging studies may support the diagnosis of RSDS, but are generally too nonspecific to make the diagnosis in the absence of clinical information. The main radiographic findings are soft tissue swelling and regional osteoporosis. This osteoporosis may be quite aggressive and severe, and may be seen in trabecular, subperiosteal, intracortical, endosteal, or subchondral bone. Some patients will present with significant juxtaarticular osteoporosis, mimicking an inflammatory arthritis such as rheumatoid arthritis. However, the absence of significant intra-articular erosions or joint space loss usually allows differentiation of RSDS from these arthropathies. Radionuclide imaging typically shows increased uptake of tracer in the articular regions, which is probably related to hyperemia. These findings may predate the clinical or radiographic findings. RSDS is generally a bilateral process, although the abnormalities are usually far more marked on one side than the other. Generally, the entire extremity distal to an affected site shows alterations, although the osteoporosis may be patchy in some cases.
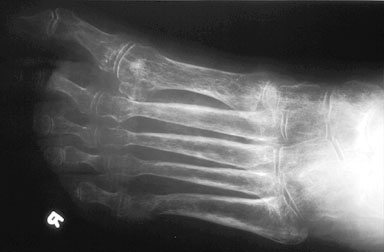
Oblique radiograph of the foot in a patient with RSDS, showing a nonspecific diffuse osteopenia throughout the foot.
Transient regional osteoporosis
Before considering this diagnosis, you should first make sure that the patient does not have any definite evidence of some predisposing cause for the osteoporosis, such as trauma or immobilization. If there is, RSDS or disuse osteoporosis would be a more likely diagnosis. However, in the absence of such predisposing causes, one may see cases of osteoporosis that are transient (self-limited and reversible) and regional in distribution. The cause for this syndrome is unknown. It generally manifests itself in one of two possibly related patterns: transient osteoporosis of the hip and regional migratory osteoporosis.
Transient osteoporosis of the hip
This is generally a unilateral process, characterized by joint pain, an antalgic limp and limited hip motion. The hip pain begins spontaneously, without an antecedent history of trauma or infection. The pain usually progresses over the next few weeks, often becoming severe enough to produce a limp. The clinical findings usually regress in 2 to 6 months without permanent sequelae. The condition may also arise in pregnant females in their third trimester. In these cases, the course is also self-limited, with full recovery evident in 3 – 12 months.
Transient osteoporosis of the hip (TOH) usually occurs in young to middle-aged adults, and is more common in men. The process is usually bilateral in men, but the left hip is almost exclusively involved in women. Radiographic findings include osteoporosis of the periarticular bone. The joint space remains normal, and fractures of the osteopenic bone may occur. Radionuclide imaging shows increased tracer uptake, which usually predate the radiographic findings. CT shows osteopenia with cortical thinning, and MRI shows findings consistent with diffuse edema in the femoral head, neck and intertrochanteric areas.
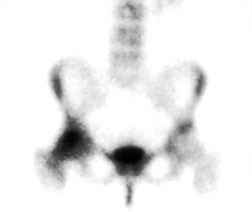
Radionuclide bone scan of a patient with TOH of the right hip shows marked uptake in the right femoral head and neck.
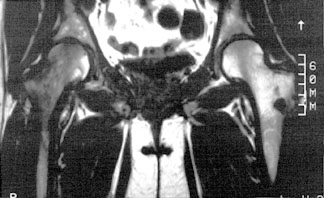
T1-weighted coronal MR of hips of the same patient with TOH shows a diffuse zone of low signal intensity in e femoral head and neck consistent with marrow edema. Compare this with the normal left femoral head and neck.
Regional migratory osteoporosis
This idiopathic disorder is characterized by local pain and swelling of the affected joint, which may be the knee, ankle, foot or hip. It is seen in men more often than women, and usually occurs in the fourth or fifth decades of life. These symptoms typically develop rapidly, last up to 9 months, and then diminish and disappear. Other joints are then affected in the same or opposite extremity. Radiographic findings of osteoporosis become apparent within weeks of months of the onset of symptoms, and are similar to those of TOH. This osteoporosis is also self-limited, and disappears following resolution of symptoms. The migratory nature of this disorder is the main factor distinguishing it from TOH.
Other causes of regional osteoporosis
Other entities may occasionally mimic transient regional osteoporosis, particularly early in their course. These include osteonecrosis, septic arthritis, rheumatoid arthritis, pigmented villonodular synovitis or idiopathic synovial osteochondromatosis. However, other findings, such as periarticular erosions or joint space narrowing will often help to distinguish these entities. The subsequent clinical course of the patient will also generally reveal the true nature of the patient’s pain.
Other causes of osteopenia
As was mentioned earlier in this chapter, osteoporosis is not the only cause of osteopenia, just the most common one. The prevalence of other causes, such as osteomalacia, hyperparathyroidism, or diffuse metastatic disease depends greatly on the exact type of patients referred to your radiologic practice. Your mileage may vary.
Osteomalacia
Osteomalacia is characterized by an abnormally high ratio of osteoid (inadequately mineralized bone matrix) to mineralized bone. Osteomalacia is a complex and protean disorder, with over 30 causes or associated diseases identified. However, for our purposes, the Cliff Notes version of osteomalacia will do. Basically, we will consider only two main causes of osteomalacia: you have your problems with vitamin D metabolism and you have your problems with renal tubular phosphate loss. That’s it. There are, of course, syndromes that fall outside of this extremely simplified approach. However, I have never seen a case of them, and don’t find their inclusion useful for most purposes.
Before we go on, let’s look at one more definition. What is rickets? It’s this: if one of these causes of osteomalacia becomes operant during childhood while the bones are still growing, the resulting abnormalities in the growing bones are called rickets.
When considering renal tubular disorders, think of just two things: X-linked hypophosphatemia and Fanconi’s syndrome. X-linked hypophosphatemia (a.k.a. familial vitamin D-resistant rickets) is the most common form of renal tubular rickets and osteomalacia.
Problems of vitamin D metabolism are a slightly more complex, and are shown in the diagram below.
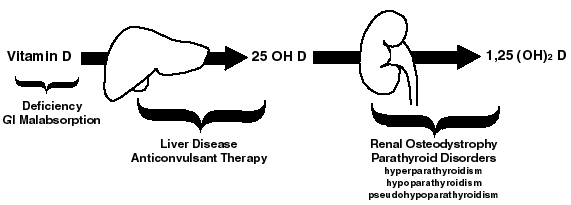
Abnormalities of vitamin D metabolism causing osteomalacia
Humans produce Vitamin D in their skin following exposure to ultraviolet light. We also obtain it from dietary sources or vitamin supplements. It is then hydroxylated by the liver to form 25 OH D, which circulates, bound to a specific binding protein. This circulating pool of 25 OH D is then further hydroxylated by the kidney as needed, to form the physiologically active form of the hormone, 1,25 (OH)2 D. As the diagram above shows, problems may occur at various stages of this process and thereby lead to osteomalacia or rickets.
Now, how do you diagnose osteomalacia or rickets? Well, it can be tricky. Some findings, such as osteopenia or coarsening of the trabeculae, are very nonspecific, and not helpful for diagnosis.
If one is dealing with a child, one can improve one’s chances of diagnosing rickets by looking closely at the fastest growing bones in the body. Therefore, look first at the costochondral junctions of the middle ribs, then the distal femur, the proximal humerus, both ends of the tibia and finally, the distal ulna and radius. Look for widened, bulky physeal plates and irregularity (“fraying”), disorganization, and splaying (“cupping”) of the bone at the junction of the metaphysis and physis. The widened growth plate is due to lack of mineralization of the cartilage matrix, and is weaker than a normal growth plate. This may predispose the patient to a “slipped” epiphysis (epiphysiolysis). If rickets begins in infancy or early childhood, the long bones will show characteristic bowing deformities. The classic “rachitic rosary” of the chest is due to cartilage overgrowth and metaphyseal splaying at the costochondral junction of the ribs.
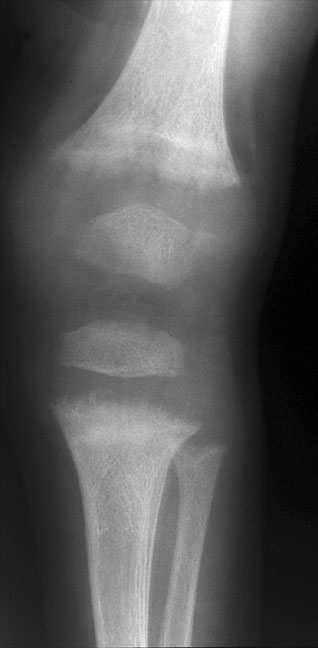
A child with rickets, demonstrating classic metaphyseal fraying and physeal widening.
The findings of osteomalacia are generally much less specific than those of rickets. Generally, all that one sees is a diffuse osteopenia, which looks just like that seen in osteoporotic patients. In some cases of osteomalacia (rare in my experience), collections of osteoid may build up to the point that these “seams” of osteoid may be seen on plain radiographs as linear lucencies oriented perpendicular to the cortical margin. If large enough, these “Looser’s zones” or pseudofractures may help lead one to the diagnosis of osteomalacia. Occasionally, one may see bowing of the long bones in an adult. In general, though, bone biopsy is far more helpful for diagnosing osteomalacia than any radiographic test.
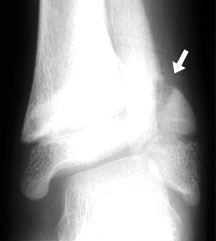
Looser zone (arrow) in the distal fibula of a child with renal osteodystrophy
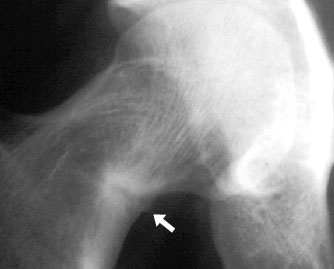
Looser zone (arrow) in the femoral neck of an adult with osteomalacia
Hyperparathyroidism
Hyperparathyroidism usually comes in two flavors: primary and secondary. The primary form is due to a hyperfunctioning parathyroid gland, usually an adenoma. However, since the advent of hemodialysis, a far more common cause for hyperparathyroidism is the secondary form, due to chronic kidney disease, especially glomerular disease. The skeletal disease seen in these patients is usually referred to as renal osteodystrophy.
The primary function of parathormone is to maintain the circulating level of calcium ion. Adequate calcium ion concentration is necessary for proper functioning of important parts of the skeletal life support system, such as the heart. Nature seems to have learned long ago how to use the skeleton not only for support, but also as a large depot of calcium. One of the major actions of parathormone is to stimulate the osteoclasts, which resorb bone and release calcium ion into the blood stream. Parathormone also acts on the small intestine to increase calcium absorption through the gut. Parathormone has an additional effect on the tubules of the kidney, where it causes phosphate excretion and calcium absorption. Both of these mechanisms lead to an increase in the serum calcium level: the former by the calcium-phosphate ion product effect and the latter directly.
Once enough bone has been resorbed from the skeleton due to elevated parathormone levels, one may see diffuse skeletal osteopenia. This finding is extremely nonspecific. However, a far more specific finding is the presence of subperiosteal resorption, which is practically pathognomonic for hyperparathyroidism. One may also see metaphyseal cutback due to the excess parathormone levels.
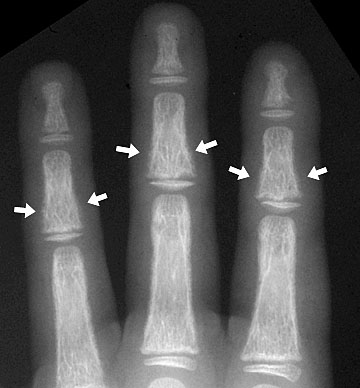
Subperiosteal resorption (arrows) in the fingers of a child with renal osteodystrophy
Sclerosis is noted adjacent to the endplates (rugger-jersey spine) in a patient with renal osteodystrophy — histologically, this represents excessive accumulation of osteoid in these areas.
The radiographic picture of renal osteodystrophy can be very confusing, since one often has overlapping findings of osteomalacia andhyperparathyroidism. In children, one may also see the rachitic findings overlapping these other two processes.
Neoplasm
Although it is uncommon, one should always remember that neoplasia can be a cause of diffuse osteopenia. In diffuse and widespread lymphoma or multiple myeloma, the radiographic appearance may be that of profound osteopenia, rather than the more usual presentation of focal lucent lesions. Certain tumors, such as oat cell carcinoma of the lung, may produce a parathormone-like substance which can lead to diffuse bone resorption and subperiosteal resorption.
This patient exhibits marked diffuse osteopenia, endplate fractures and pathologic compression fractures due to widespread mulitiple myeloma
Wise Sayings
- Remember “Resnick’s Rule”: “You can take a normal patient and an abnormal X-ray technologist and produce osteoporosis at will.”
- In cases of widespread, really terrible osteoporosis, consider multiple myeloma, especially if the patient seems a bit too young to have postmenopausal or senile osteoporosis.
References
- Harris WH, Heaney RP. Skeletal renewal and metabolic bone disease. N Engl J Med 1969;280:193.
- Lutwak L, Whedon GD. Osteoporosis. DM April 1963, page 1.
- Resnick D. Personal communication.