UW Radiology
Radiochemistry Analytics
The radiopharmaceutical formulated dose is subjected a range of analytics to ensure quality control and reproducibility of your research study.
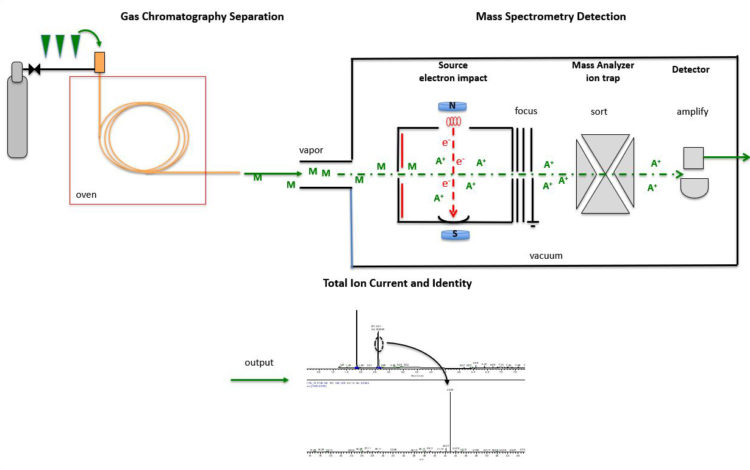
The formulated dose is injected onto GCMS, to ensure organic solvents have been removed to levels below USP allowable limits.
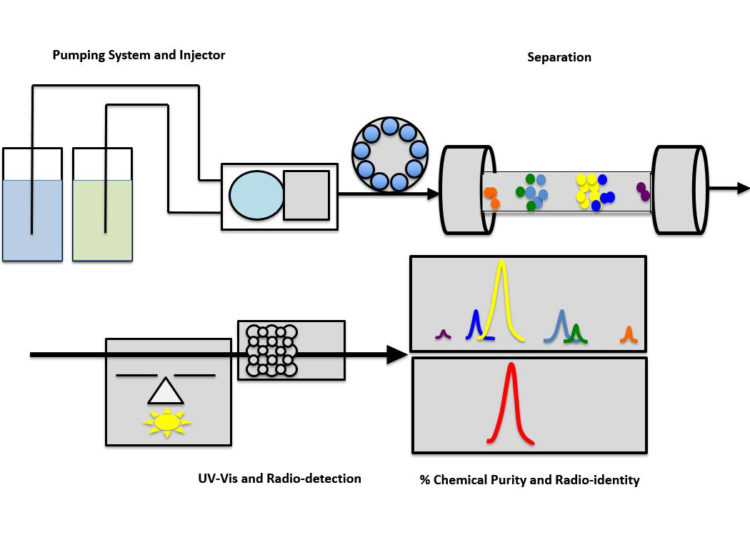
The formulated radiopharmaceutical dose is injected onto analytical HPLC to confirm radio-identity, and to quantify both product level and the level of impurities, including remaining precursor.
Additional analytics include:
- Gamma radiation counting confirms radionuclide identity for all patient dose material.
- Other analytics include confirmation of sterility of the patient dose, including endotoxin level