About Us
Cine from the heart
Our Cardiac and Body Advanced Clinical Research Imaging group consists of clinician scientists and basic scientists in the field of radiology working to enhance interpretation and analysis of MRI data for early diagnosis and to better inform treatment strategies of diseases that affect the heart and the abdominal organs.
Research Goals
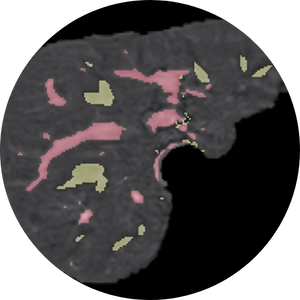
Image from liver segmentation
Our work focuses on cardiac and abdominal organs pathologies; specifically, clinical and translational imaging of the heart, great vessels, liver, and prostate. Our main research goal is to investigate the value of new advanced imaging biomarkers to improve patient care, by using novel quantitative non-invasive imaging approaches, such as advanced diffusion-weighted, 3D cardiac function and contrast-enhanced MRI techniques. Further, our work relies on the application of advanced imaging post-processing tools like MR strain and radiomics analysis, and deep learning automation strategies to optimize workflow, diagnostic precision and accuracy.
Who We Are
Visit our Member Biographies page to learn more about our lab group.

Antonio Westphalen, M.D., Ph.D.

Karen Ordovas, M.D., MAS

Anna Naumova, Ph.D.

Hamid Chalian, M.D.

Guilherme Moura Da Cunha, M.D.

Mohamed Abdelmotleb, M.D.

Avanti Gulhane, M.D., DNB, FSCMR

Ali Nabipoor, M.D.

Negar Firoozeh, M.D.
Active Studies
Strain Analysis and Radiomics Analysis as a Novel Post-processing Technique for Detecting Inflammation with CMR in Cardiac Sarcoidosis
In this study, we are trying to use strain values and radiomics features as a new biomarker to diagnose inflammation in cardiac sarcoidosis. Currently, CMR and PET scans are the diagnostic modality of choice for diagnosing fibrosis and inflammation in cardiac sarcoidosis, respectively. The result of this study has the potential to substitute PET scan with CMR for diagnosing inflammation. Ultimately, a one stop shop assessment with CMR would be beneficial for optimizing resources and patient care.
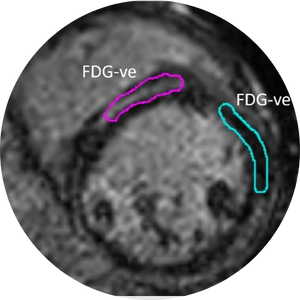
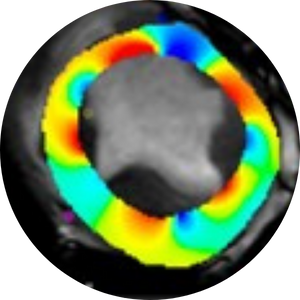
Radiomics and Strain Analysis of CMR in Animal Models for Quantitative Functional Assessment of Post-Infarction Myocardium Before and After Cell Therapy
This study is trying to find novel quantitative CMR biomarkers which are able to track the infarcted heart regeneration after transplantation of therapeutic cells in animal models. The final goal is to be able to follow the recovery period in human patients receiving cell transplants.
Radiomics Analysis as a Novel Post-processing Technique for Detecting Transitional Zone Prostate Cancer on MRI
Transitional zone prostate cancers are harder to detect with examination and imaging studies due to their location and transitional zone tissue composition, respectively. This study aims to extract radiomics features as a new biomarker to diagnose transitional zone prostate cancer.
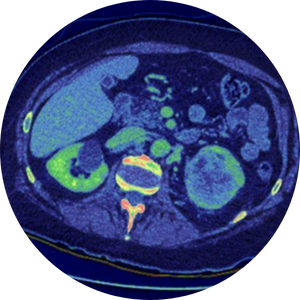
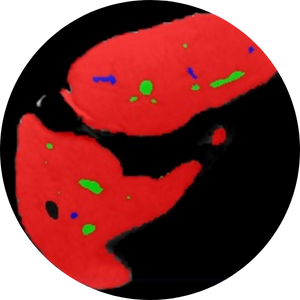
Deep learning-based organ segmentation
Organ segmentation (i.e., delineation of an organ of interest on medical images) allows for quantitative assessment of organ volume, function, and disease severity. Yet, manual organ segmentation is a labor-intensive, time-consuming process that hinders its routine application in clinical care and in the research setting. Deep learning is a technical advance under the umbrella term of Artificial Intelligence (AI) that allows computers to learn how to perform objective tasks on datasets such as radiology images. The use of deep learning will allow for the routine use of organ segmentation for quantitative analysis of MRI images in large datasets, such as for population screening of disease or on multiple time-point imaging studies for longitudinal assessment of disease progression or response to treatment.
Mitigation of Calcium Blooming Artifact in CCTA
We are developing novel imaging techniques and protocols to improve the accuracy of CCTA in assessment of the coronary artery plaques by decreasing calcium blooming artifact. We are developing a library of coronary phantoms to test novel technologies that improve coronary plaque characterization.
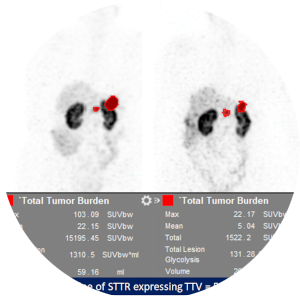
Outcomes Assessment of Patients with Neuroendocrine Neoplasms
Retrospective observational study to establish imaging metrics on interim and restaging scans that can predict response and clinical outcome in patients treated with 177Lu-DOTATATE PRRT.
10-year database for PSMA PET imaging and therapy in patients with prostate cancer
The aim is to understand how PSMA PET data can be used to optimize treatment decisions and outcomes for patients with prostate cancer. We will generate a database that will include all clinical information, imaging metrics, management, and treatment outcomes in patients with prostate cancer that underwent conventional imaging (CT/MRI/Bone scan/ F18-Fluciclovine (Axumin)) and PET scan with 68Ga-PSMA-11, F18-DCFPyL (Pylarify), and/or its treatment with 177Lu-PSMA-617.
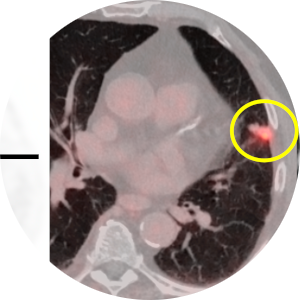
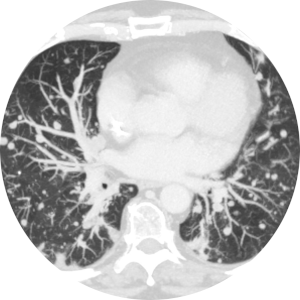
Assessment of the Growth Kinetics of Lung Cancer Post-Treatment
Understanding the growth pattern of lung tumors post treatment is even more important after introduction of new treatment options for lung cancer patients. We are planning to investigate the changes in lung tumors over the course of treatment in a retrospective observational study. In this project lung tumor volume (3D) and CT features will be assessed at different time points through the treatment (chemotherapy, radiotherapy, or combination of both) course.
Improving the Role of CT in Assessment of the Structural Heart Diseases
Structural heart diseases include any abnormality in the heart's muscle, chambers, vessels, and valves. Role of cardiac CT in assessment of structural heart disease and pre intervention planning is rapidly growing. So, in this project, we will focus on improving CT scan acquisition, post-processing, analysis method (qualitative and quantitative), and imaging protocols to enhance the diagnostic accuracy of this growing imaging modality for clinical diagnosis of structural heart abnormalities.
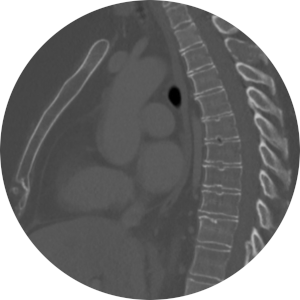
68Ga-PSMA-11 PET in Patients with Prostate Cancer
Determine rate of overall changes in management by comparing planned management strategy using conventional imaging with executed management strategy incorporating information from 68Ga-PSMA-HBED-CC (68Ga-PSMA-11) positron emission tomography/computed tomography (PET/CT), regardless of treatment modality.
Copyright © 2025 University of Washington | All rights reserved
