Our Imaging Technology
Imaging Technique Overview
MRI Overview
Magnetic Resonance Imaging (MRI) overcomes the limitations of imaging methods such as CT and x-ray imaging that are based solely on the measurement of attenuation of x-rays as they pass through tissues. MRI is an essential research tool for the non-invasive study of both the biological structures and functions of the human body. MRI can be used to understand disease processes and to develop novel treatment approaches. Human MRI studies allow for unique view of virtually every organ of the body. From anatomic study to functional imaging evaluation and from imaging alone to imaging guided HIFU therapy, the use of innovative MRI methods has revolutionized biomedical sciences. MRI has multidimensional and multi-contrast capabilities, in depiction of anatomic information. The BMIC is equipped to collect a variety of data types, both qualitative and quantitative. Several data acquisition techniques are described below.
- Measure anatomy (Conventional Structural MRI),
- Measure arterial and venous blood flow velocity (MR Angiography including Phase contrast MRI),
- Monitor brain function during the performance of a specific task (functional MRI),
- Measure the concentration of tissue metabolites in vivo (MR Spectroscopy),
- Measure cerebral blood flow (Perfusion MRI)
- Observe diffusion of water molecules through brain tissue (Diffusion-weighted MRI),
- T1/B1 measurements
- Cross relaxation imaging
Conventional Structural MRI
In clinical practice, MRI is primarily used for the acquisition of structural images that provide views of internal structures. Our research MR system is capable of acquiring such images. Structural images are used by researchers for the establishment of diagnoses, measurement of tissue characteristics, and monitoring the effects of treatment. Structural MRI can also serve as references for localized spectroscopy.
MR Angiography
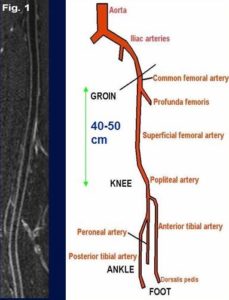
MR angiography (MRA) provides a clear view of blood vessels throughout the body non-invasively. MRA techniques use physical properties of blood flow to selectively enhance the appearance of arteries (arteriography) and veins (venography). Conventional MRA often does not require injection of a contrast agent prior to imaging. Contrast-enhanced MRA can be used in select situations to provide detailed vessel information useful in research with specific vascular interests and needs. Black-blood MRA technique developed by our MR physicists not only provides blood vessel luminal information, but also vessel wall characteristics such as presence of intraplaque hemorrhage. The time efficient and larger anatomic coverage allows for fast evaluation of carotid and intracranial arteries for stroke patients. The newly developed techniques by our MR physicists included 3D MERGE and SNAP. 3D MERGE allows for isotropic volumetric depiction of vascular beds for location of atherosclerotic plaques. SNAP allows for simultaneous identification of intraplaque hemorrhage and vascular luminal stenosis (Niranjan Balu, PhD, VIL, and Jinnan Wang, PhD, Philips Healthcare).
Functional MRI
With functional MRI (fMRI), rapid dynamic images are acquired at a rate of approximately one image every 100 milliseconds. The images are used to derive information about regional changes in blood flow and blood oxygenation to identify areas of the brain activities associated with specific tasks. This non-invasive technique requires no injection and is highly sensitive to the brain’s response to motor, sensory and cognitive tasks. With this technique, investigators utilize fMRI to study a variety of neurodevelopmental and neurodegenerative disorders, including dyslexia, autism, HIV dementia, fetal alcohol syndrome, traumatic brain injury, stroke and multiple sclerosis.
MR Spectroscopy
Magnetic resonance spectroscopy (MRS) allows scientists to detect the concentration of a variety of biological metabolites. MR system is used to perform these measurements in very specific locations in the body for sample sizes down to the order of approximately 1 cubic centimeter of tissue in vivo. Techniques have also been developed to calculate the spatial distribution of different metabolites in the brain.
Perfusion Imaging
MR can also be used to generate images that measure blood flow through the brain capillaries, also known as tissue perfusion. This can be done using either a contrast agent or with a non-invasive MR technique called arterial spin labeling (ASL).
Diffusion Tensor Imaging (DTI)
Diffusion tensor imaging (DTI) is a MRI technique that enables the measurement of the restricted diffusion of water molecules in tissue. The principal application is in the imaging of white matter where the location and orientation of the tracts can be measured. The architecture of the axons in parallel bundles and their myelin sheaths facilitates the diffusion of the water molecules preferentially along the dominant direction. Such preferentially oriented diffusion is called anisotropic diffusion. Scientists have used DTI to study white matter fiber tract differences between dyslexic and normal readers.
T1/B1 Measurements
A new time-efficient and accurate technique for simultaneous mapping of T1 and B1 is proposed based on a combination of the actual flip angle (FA) imaging and variable FA methods. Variable FA-actual FA imaging utilizes a single actual FA imaging and one or more spoiled gradient-echo acquisitions with a simultaneous nonlinear fitting procedure to yield accurate T1/B1 maps. The advantage of variable FA-actual FA imaging is its high accuracy at either short T1 times or long repetition times in the actual FA imaging sequence. Simulations show this method is accurate to 0.03% in FA and 0.07% in T1 for ratios of repetition time to T1 time over the range of 0.01-0.45. It is sufficient to use only one small FA spoiled gradient-echo acquisition in brain imaging, which reduces spoiling requirements and scan time, as compared to the original variable FA method. In vivo validation yielded high-quality 3D T1 maps and T1 measurements within 10% of previously published values and within a clinically acceptable scan time. The variable FA-actual FA imaging method will increase the accuracy and clinical feasibility of many quantitative MRI methods requiring T1/B1 mapping such as dynamic contrast enhanced perfusion and quantitative magnetization transfer imaging (Hurley SA, Yarnykh VL, Johnson KM, Field AS, Alexander AL, Samsonov AA. Magn Reson Med 2012; 68:54-65).
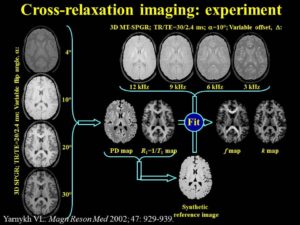
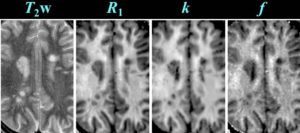
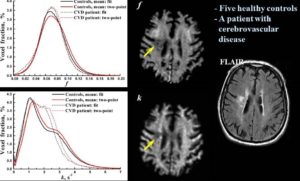
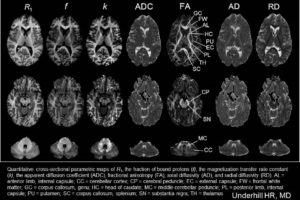
Cross-relaxation Imaging (CRI)
Cross-relaxation imaging (CRI) describes the magnetization transfer within tissues between mobile water protons and macromolecular protons. Whole-brain parametric maps of the principle kinetic components of magnetization transfer, the fraction of protons (f) and the rate constant (k), reveal detailed anatomy of white matter (WM) fiber tracts. In WM, neither f nor k was significantly correlated to fractional anisotropy (FA), radial diffusivity (RD), and axial diffusivity (AD). In contrast, both f (r=0.90 and r=-0.80) and k (r=0.92 and r=-0.89) in gray matter (GM) were strongly correlated to FA and RD, respectively. A moderate correlation between apparent diffusion coefficient (ADC) and k (r=0.48) was identified in WM, while an inverse correlation was identified in GM (r=-0.72). The lack of association between CRI and FA in WM is consistent with differences in the underlying physical principles between techniques – fiber density vs. directionality, respectively. The association in GM may be attributable to variable axonal density unique to each structure. Whole-brain CRI provides distinct quantitative information compared to DTI, and CRI parameters may prove constructive as biomarkers in neurological diseases (Drs Hunter Underhill, Chun Yuan, and Vasily Yarynkh).
These exciting methods are allowing scientists to non-invasively probe the inner structures and functions of the human body or animals in more detail. Moreover, the potential for future growth in the capabilities of MRI and the beneficial effects on science and medicine is seemingly endless.
BMIC HUMAN RESEARCH HIGHLIGHTS
1. Vascular MR Imaging
- High Resolution Multi-contrast Weighted MR Imaging of Carotid Atherosclerosis.
Intraplaque hemorrhage in the right common carotid artery is demonstrated by hyperintense signals on the 3D TOF, T1W, and IR TFE sequences. Hypointense signals in both the TOF and IR TFE images are characteristic of regions with dense calcification. MR images were acquired on our 3T Philips scanner along with the use of our 8-element phased array carotid coil (Chu B, et al. MRI features of the disruption-prone and the disrupted carotid plaque: a pictorial essay. JACC Cardiovasc Imaging 2009; July;2(7):883-896).
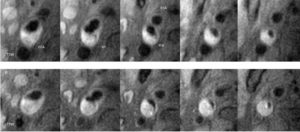
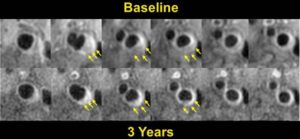
- Intraplaque Hemorrhage is Associated with Rapid Progression of Carotid Atherosclerotic Plaque.
Representative T1-weighted images demonstrate rapid progression of atherosclerosis with intraplaque hemorrhage in the right carotid artery. Each column presents matched cross sectional locations in the carotid artery from baseline (upper row) and 18 months later (lower row). A marked decrease in lumen area and an increase in wall area are present in each location of the second examination. MR images were acquired on a 1.5 T G.E. Signa scanner along with the use of a 4-element phased array carotid coil. CCA = Common Carotid Artery; Bif = Bifurcation; ICA= Internal Carotid Artery; and ECA = External Carotid Artery (Takaya N, et al. Circulation. 2005 May 31;111(21):2768–75).
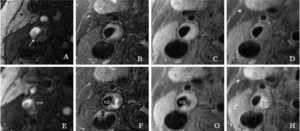
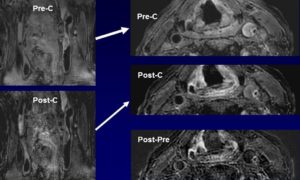
- Multi-contrast Weighted MRI in the Identification of Carotid Artery Ulceration.
Matched baseline images (A, 3-dimensional time of flight; B, T2-weighted; C, pre–contrast-enhanced, T1-weighted; and D, post–contrast-enhanced, T1-weighted), obtained by high-spatial-resolution MRI (matrix, 256×256; field of view, 160×120 mm; slice thickness, 2 mm), demonstrate carotid atherosclerotic plaque and luminal stenosis in the right internal carotid artery. A dark band between the lumen and arterial wall (arrow, A) suggests intact fibrous cap. Hyperintense signal on images A, B, and C suggests presence of intraplaque hemorrhage. Follow-up MR images E through G (corresponding to images A through C, respectively, in sequence and location) demonstrate surface disruption (open arrows) and penetrating ulcer into the plaque. The signal within the ulcer demonstrates hyperintense signal on E, mixed hyperintense and hypointense signal on F, hypointense signal on G, and contrast enhancement on H. This signal pattern suggests turbulent flow within the ulcer. Note slight location mismatch between post–contrast-enhanced T1-weighted images at baseline (D) and follow-up (H). This mismatch resulted from the patient’s minimum motion during image H acquisition (Chu B, et al. Serial High-Spatial-Resolution, Multisequence Magnetic Resonance Imaging Studies Identify Fibrous Cap Rupture and Penetrating Ulcer Into Carotid Atherosclerotic Plaque. Circulation.2006; 113: e660-e661).
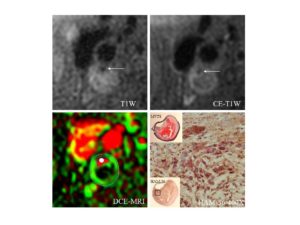
- Carotid Plaque Inflammation Identified by MR DCE.
Plaque with histologically confirmed inflammation of the fibrous cap shows a strong enhancement pattern by dynamic contrast-enhanced MRI (DCE-MRI), with high Ktrans (green) in the adventitial vasa vasorum (near the outer vessel wall boundary) and along the lumen boundary (inner boundary demarcating red lumen region). Movat’s Pentachrome stains hemorrhage within the lipid-rich necrotic core red. Immunocytochemistry with HAM 56, an antibody to human macrophages, shows an abundance of macrophages in the fibrous cap. Arrows indicate the stenotic lumen of the internal carotid artery. DCE-MRI was collected at 10 time points, with a separation interval of 15 seconds between time points. Gadolinium-based contrast agent (Omniscan; GE Medical Systems; 0.1 mmol per kilogram body weight) was injected at a rate of 2 mL/sec. All MR images were acquired on a 1.5 T G.E. Signa scanner along with the use of a 4-element phased array carotid coil (Kerwin, WS, et al. Quantitative magnetic resonance imaging analysis of neovasculature volume in carotid atherosclerotic plaque. Circulation, 107 (2003). 851–856).
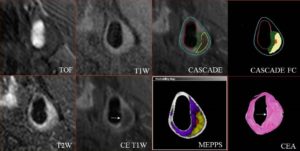
- Image Post-processing: CASCADE and MEPPS
Quantification of in vivo MR images (3D TOF, T1W, T2W, and CE-T1W utilizing CASCADE (Computer-Aided System for Cardiovascular Disease Evaluation) to generate component outlines. CASCADE FC is an additional algorithm that collects fibrous cap length, depth, and area. The automated map of the plaque is produced by MEPPS (Morphology Enhanced Probability maPS). Loose matrix is shown in purple, lipid-rich necrotic core in yellow, and intraplaque hemorrhage in red on the MEPPS image. Histology from carotid endarterectomy (CEA) confirms the components and the enhancing thick fibrous cap (arrow). Invivo MR images were acquired on our 3T MR scanner (Chu B, et al. MRI features of the disruption-prone and the disrupted carotid plaque: a pictorial essay. JACC Cardiovasc Imaging 2009; July;2(7):883-896).
- Carotid Atherosclerotic Plaque Regression Following Lipid-Lowering Therapy.
Significant lipid content reduction (yellow arrows) and plaque regression at 3 years compared to baseline in the left carotid artery. Overall, 11% of study subjects had completed plaque lipid depletion over 3 years (Zhao XQ, et al. MR Imaging of Carotid Plaque Composition During Lipid-Lowering Therapy : A Prospective Assessment of Effect and Time Course. JACC: Cardiovascular Imaging. 2011;4(9): 977–986).
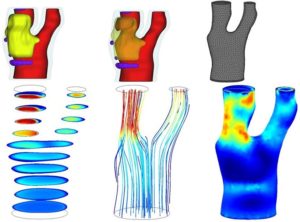
- Computational Fluid Dynamics (CFD) reproduce blood flow through reconstructed geometry of the carotid bifurcation.
The top panel shows a 3D reconstruction of a diseased carotid artery. The plaque extends from the bifurcation into the internal carotid artery. Plaque components include a lipid-rich necrotic core (yellow) with intraplaque hemorrhage (as shown in dark orange in B) and loose matrix (purple). A computational mesh (C) was built based on the arterial lumen (red). The bottom panel shows the result of the CFD simulation. Panel D shows the flow velocity field for various cross-sectional slices. The corresponding flow streamlines are shown in E. The internal carotid artery is subjected to higher wall shear stress forces than the external carotid artery (F). (Canton G, Yuan C).
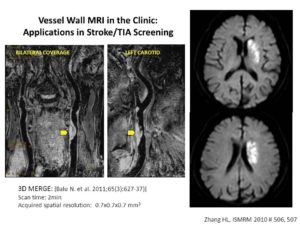
- Novel Black-Blood Vessel Wall MR Technique: 3D MSDE Prepared Rapid Gradient Echo (3D MERGE).
Black-blood MRI is a promising tool for carotid atherosclerotic plaque burden assessment and compositional analysis. However, current sequences are limited by large slice thickness. Accuracy of measurement can be improved by moving to isotropic imaging but can be challenging for patient compliance due to long scan times. We present a fast isotropic high spatial resolution (0.7×0.7×0.7 mm3) three-dimensional black-blood sequence (3D-MERGE) covering the entire cervical carotid arteries within 2 min thus ensuring patient compliance and diagnostic image quality. The sequence is optimized for vessel wall imaging of the carotid bifurcation based on its signal properties.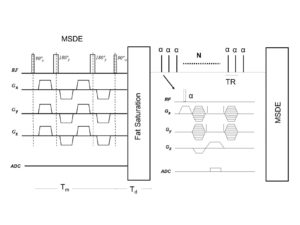
3D MERGE isotropic property allows multi-plane reformatting capability.
Quantitative plaque morphology measurements and signal-to-noise ratio measures show that 3D-MERGE provides good blood suppression and comparable plaque burden measurements to existing MRI protocols.
3D MERGE can be used in the identification of cause for ischemic stroke or TIA Screening.
3D-MERGE is a promising new tool for fast and accurate plaque burden assessment in patients with atherosclerotic plaque (Niranjan Balu, Vasily Yarnykh, Jinnan Wang, Thomas Hatsukami, Chun Yuan. Magn Reson Med. 2011;65:627-37).
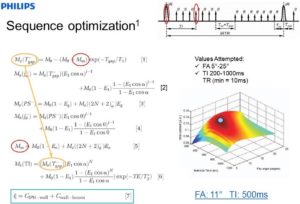
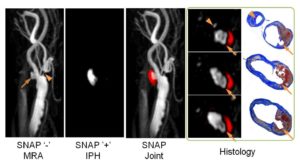
- Sequence Optimization on Simultaneous Non-contrast Angiography and Intraplaque Hemorrhage (SNAP) Imaging for Atherosclerosis.
SNAP For Simultaneous Identification of Carotid Artery Lumen and Intraplaque Hemorrhage.
SNAP MR imaging technique is proposed to detect both luminal stenosis and hemorrhage in atherosclerosis patients in a single scan. The SNAP sequence utilized a phase sensitive acquisition, and was designed to provide positive signals corresponding to intraplaque hemorrhage and negative signals corresponding to lumen. SNAP images were compared to time-of-flight images to evaluate lumen size measurements using linear mixed models and the intraclass correlation coefficient. Intraplaque hemorrhage identification accuracy was evaluated by comparing to magnetization-prepared 3D rapid acquisition gradient echo images using Cohen’s Kappa. Quantitatively, the lumen size measurements by SNAP were strongly correlated (intraclass correlation coefficient = 0.96, P < 0.001) with those measured by time-of-flight. For intraplaque hemorrhage detection, strong agreement (κ = 0.82, P < 0.001) was also identified between SNAP and magnetization-prepared 3D rapid acquisition gradient echo images. SNAP imaging technique shows great promise for imaging both lumen size and carotid intraplaque hemorrhage with a single scan (Wang J, et al. Simultaneous noncontrast angiography and intraplaque hemorrhage (SNAP) imaging for carotid atherosclerotic disease evaluation. Magn Reson Med. 2013 Feb;69(2):337-45).
2. Cardiac MR Imaging
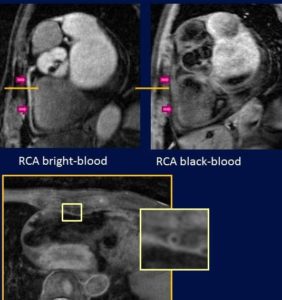
- Coronary Artery Bright- and Black-Blood MR Angiography.
- Black-blood MR Imaging of Femoral Artery Allowing For Visualization of Vessel Wall.
3. Neuroimaging
- Cross-relaxation MR Imaging.
A new method of pulsed Z-spectroscopic imaging is proposed for in vivo visualization and quantification of the parameters describing cross-relaxation between protons with liquid-like and solid-like relaxation properties in tissues. The method is based on analysis of the magnetization transfer (MT) effect as a function of the offset frequency and amplitude of a pulsed off- resonance saturation incorporated in a spoiled gradient-echo MRI pulse sequence. The theoretical concept of the method relies on an approximated analytical model of pulsed MT that provides a simple three-parameter equation for a pulsed steady-state Z-spectrum taken far from resonance. Using this model, the parametric images of cross-relaxation rate constant, content, and T(2) of the semisolid proton fraction can be reconstructed from a series of MT-weighted images and a coregistered T(1) map. The method provided high-quality 3D parametric maps within an acceptable scanning time. The estimates of cross-relaxation parameters in brain tissues were shown to be quantitatively consistent with the literature data. Clinical examples of the parametric images of human brain pathologies (multiple sclerosis and glioma) demonstrated high tissue contrast and clear visualization of the lesions (Yarnykh VL. Pulsed Z-spectroscopic imaging of cross-relaxation parameters in tissues for human MRI: theory and clinical applications. Magn Reson Med. 2002 May;47(5):929-39).
- Cross-relaxation MR Imaging in the Application of Multiple Sclerosis.
- Cross-relaxation MR Imaging in the Application of Stroke.
- Quantitative Cross-sectional Parametric Maps.
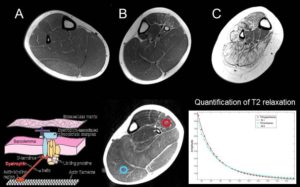
4. Musculoskeletal Imaging
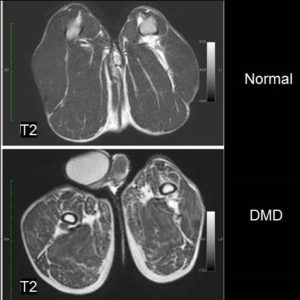
- Human Duchenne Muscular Dystrophy (Drs. Parnell, Friedman, Shaw -Seattle Children’s).
- Human Fascioscapulohumeral Muscular Dystrophy (FSHMD) (Drs. Shaw/Friedman –Pacific Northwest Friends Foundation Grant).
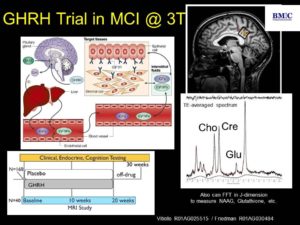
5. MR spectroscopy
BMIC ANIMAL RESEARCH HIGHLIGHTS
- Cardiac Imaging
- Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts.
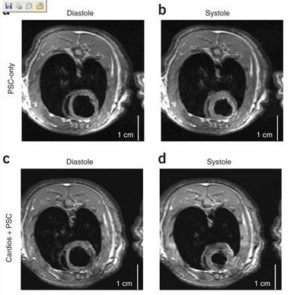
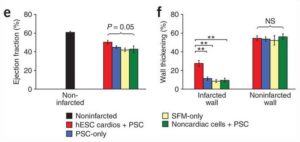
Cardiomyocytes derived from human embryonic stem (hES) cells potentially offer large numbers of cells to facilitate repair of the infarcted heart. However, this approach has been limited by inefficient differentiation of hES cells into cardiomyocytes, insufficient purity of cardiomyocyte preparations and poor survival of hES cell-derived myocytes after transplantation. Seeking to overcome these challenges, we generated highly purified human cardiomyocytes using a readily scalable system for directed differentiation that relies on activin A and BMP4. We then identified a cocktail of pro-survival factors that limits cardiomyocyte death after transplantation. These techniques enabled consistent formation of myocardial grafts in the infarcted rat heart. The engrafted human myocardium attenuated ventricular dilation and preserved regional and global contractile function after myocardial infarction compared with controls receiving noncardiac hES cell derivatives or vehicle. The ability of hES cell-derived cardiomyocytes to partially remuscularize myocardial infarcts and attenuate heart failure encourages their study under conditions that closely match human disease (Laflamme MA, Naumova AV, et al. Nat Biotechnol. 2007;26:1015-24).
- Stem Cell Transplantation.
Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts (Laflamme MA, Naumova AV, et al. Nat Biotechnol. 2007;26:1015-24).
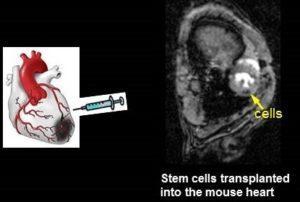
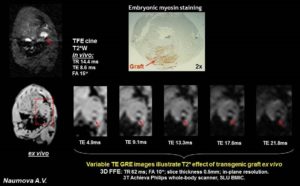
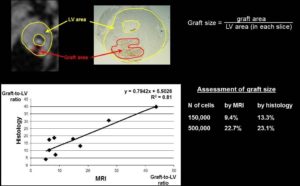
- Ferritin Overexpression for Molecular Imaging of Transplanted Cells.
An unmet need in cardiac cell therapy is a noninvasive imaging technique capable of tracking changes in graft size over time and monitoring cell dynamics such as replication and death, factors to which commonly used superparamagnetic nanoparticles are insensitive. Our goal was to explore if overexpression of ferritin, a nontoxic iron-binding protein, can be used for noninvasive magnetic resonance imaging (MRI) of cells transplanted into the infarcted heart. Mouse skeletal myoblasts (C2C12 cells) were engineered to overexpress ferritin. Ferritin overexpression did not interfere with cell viability, proliferation, or differentiation into multinucleated myotubes. Ferritin overexpression caused a 25% decrease in T2 relaxation time in vitro compared to wild-type cells. Transgenic grafts were detected in vivo 3 weeks after transplantation into infarcted hearts of syngeneic mice as areas of hypointensity caused by iron accumulation in overexpressed ferritin complexes. Graft size evaluation by MRI correlated tightly with histologic measurements (R2 = .8).
Our studies demonstrated the feasibility of ferritin overexpression in mouse skeletal myoblasts and the successful detection of transgenic cells by MRI in vitro and in vivo after transplantation into the infarcted mouse heart. These experiments lay the groundwork for using the MRI gene reporter ferritin to track stem cells transplanted to the heart (Naumova AV, Reinecke H, Yarnykh V, Deem J, Yuan C, Murry CE. Mol Imaging. 2010 Aug;9(4):201-10).
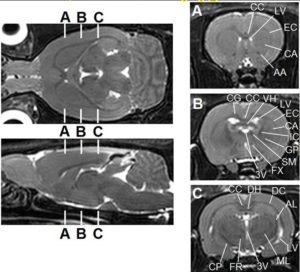
- In vivo Whole Brain Rat MR Imaging at 3.0T at BMIC (Dr. Hunter Underhill)
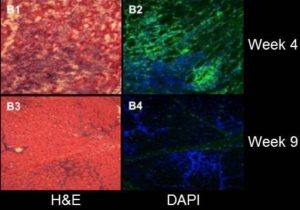
- Dog Duchenne Muscular Dystrophy at 3.0T MRI at BMIC (Drs. Donghoon Lee, Martin Kushmerik, Zejing Wang, and Stephen Tapscott)
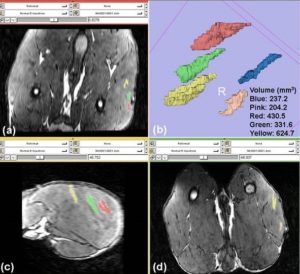
- AAV Vector Volume Measured by 3D MRI (Drs. Donghoon Lee, Martin Kushmerik, Zejing Wang, and Stephen Tapscott)
Wang Z, Storb R, Lee D, Kushmerick MJ, Chu B, Berger C, Arnett A, Allen J, Chamberlain JS, Riddell SR, Tapscott SJ. Immune responses to AAV in canine muscle monitored by cellular assays and noninvasive imaging. Mol Ther. 2010 Mar;18(3):617-24.