Robert Miyaoka, Ph.D., and Larry Pierce, Ph.D., awarded National Institute of Health grant

Robert Miyaoka, Ph.D.
Research Professor Robert Miyaoka, Ph.D., and Research Scientist Larry Pierce, Ph.D., were recently awarded a grant from the National Institute of Health to initiate translation of a new imaging technology they invented to a clinical setting.
The new imaging technology seeks to revolutionize nuclear medicine and single photon emission computed tomography (SPECT) by enabling a >2000 times gain in sensitivity (versus current clinical SPECT cameras), as well as 100-fold improvement in volumetric image resolution.
Drs. Miyaoka and Pierce were awarded the NIH STTR Phase I grant, “Ultra-high sensitivity, high spatial resolution single photon emission tomography using mechanical flux manipulation (MFM).” The development of this new technology is a joint effort between Precision Sensing, LLC, founded by the pair of doctors, and the University of Washington Department of Radiology.
During a casual conversation in Feb. 2020, Dr. Pierce told Dr. Miyaoka about his idea of a collimator-less SPECT camera and a new methodology for image reconstruction. His new image reconstruction method was based upon flux analysis recorded for each element of a large pixelated detector and is vastly different than all currently used methods in nuclear medicine. During the discussion, Dr. Miyaoka suggested adding a moving attenuator wand between the patient being imaged and the detector — the movement of the wand combined with the flux analysis could lead to better image resolution.
That weekend, Dr. Pierce worked on his math algorithms and by Monday enthusiastically told Dr. Miyaoka that he was certain that the methodology would work. They immediately submitted a record of invention (ROI) to CoMotion, the University of Washington’s Technology Transfer Office, and began to seek grant funding to build the first prototype system to experimentally prove the concept.
In addition to the NIH STTR Phase I grant, the duo was also recently awarded a Washington Entrepreneurial Research Evaluation & Commercialization Hub (WE-REACH) grant to further support the commercialization of their technology. These grants will allow Drs. Pierce and Miyaoka to build and characterize the performance capabilities of the first experimental prototype MFM imaging system.
What this means for the future of SPECT imaging:

Larry Pierce, Ph.D.
While there have been dramatic improvements in image quality during the last 20-30 years in almost all other areas of radiologic imaging (PET, MRI, CT, Ultrasound and even X-ray), image quality improvements in SPECT imaging have been relatively modest. This is largely because the core components of most clinical nuclear medicine and SPECT imaging systems are the same as the elements used when the gamma camera was first introduced by Hal Anger in the 1950s. These components include a collimator, a detector and an array of photomultiplier tubes. Bone scans, a standard imaging procedure in nuclear medicine, largely look the same today as they did 20-30 years ago due to the gamma camera’s dependence on the collimator as a necessary component to create an image.
A standard gamma camera collimator has an efficiency of 0.04%. Instead of using a collimator, the new type of nuclear medicine camera called MFM has an attenuating wand with an efficiency of
>50%. By adding more detectors, the sensitivity can be boosted even more.
In addition to eliminating the collimator (“the weak link of current technology,” Dr. Miyaoka said), the researchers have developed a new methodology for producing an image from the collected
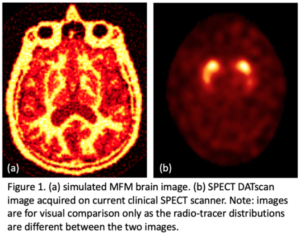
data. By moving their attenuating wand in specific patterns between the patient and the imaging detector differential flux patterns are induced that can be reconstructed to produce a three-dimensional image of the activity distribution in a patient. In addition to the huge gains in sensitivity, initial simulation results are showing a greater than 100-fold improvement in volumetric image resolution between images produced using MFM versus standard gamma camera SPECT systems (see Figure 1 for comparison).