First-in-Human PET/CT imaging study to evaluate [11C]-rosuvastatin hepatobiliary clearance and drug-drug interactions at University of Washington Medical Center
On May 7th, 2018 a ‘first-in-human’ PET imaging study with a novel radiotracer [11C]-rosuvastatin was completed in UWMC Radiology as part of an inter-departmental clinical research project led by Professor Jash Unadkat (Dept. of Pharmaceutics). The study aims to validate whether human hepatobiliary transport and hepatic concentrations of a drug (in this case [11C]-rosuvastatin) may be predicted from in-vitro data.
The Unadkat laboratory has developed in-vitro methods using transporter-expressing cell lines, human hepatocytes and quantitative proteomics to predict transporter-based in-vivo hepatobiliary drug clearance (http://bit.ly/2Lwhhx0). Improved prediction of human hepatobiliary clearance and tissue concentrations of drug will help the pharmaceutical industry expedite the development of drugs for clinical trials, understand how genetics affect the way individuals process medications and importantly also study the impact of co-medications, i.e. drug-drug interactions.
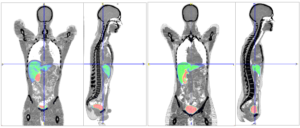
PET/CT images of [11C]-rosuvastatin uptake in a male and a female volunteer. Coloured regions show areas of [11C]-rosuvastatin accumulation measured by PET. Grey-scale images are from the x-ray CT scan and show anatomy. Images courtesy of Mark Muzi.
Rosuvastatin (a cholesterol lowering drug routinely used in drug development studies) was selected as the model drug since hepatobiliary transport is the dominant clearance pathway and it is eliminated from the body mostly as the unchanged drug. For this study, rosuvastatin was radiolabelled with the isotope carbon-11, a positron emitter with a 20 min half-life that can be imaged using positron emission tomography (PET). PET scanners are combined with x-ray computed tomography (CT) scanners to provide both molecular (PET) and anatomical (CT) images as shown in the figures, which demonstrate expected accumulation in the liver, gall bladder and the urinary bladder. This approach allows measuring the hepatic uptake clearance, hepatic concentrations, and biliary clearance of [11C]-rosuvastatin. These studies are being done in the absence and presence of cyclosporine (a polypeptide transport uptake inhibitor) as a first step in evaluating drug-drug interactions.
Dept. of Radiology collaborators include Dr. Steven Shoner and the radiochemistry staff, Drs. David Lewis and Jean Lee, Mark Muzi and Jeremy Iman, Missy Wanner, and the PET/CT clinical imaging technologists. Other collaborators are Dr. Scott Lee and staff from the School of Medicine; Dr. Matthew Pennington from the Dept. of Anaesthesiology & Pain Medicine, and Dr. Sarah Billington and other members of the Unadkat laboratory. The study is supported through UWRAPT, a consortium funded by Genentech, Gilead, Biogen, Merck, Takeda, BMS and Pfizer.
For further information:
Dept. of Pharmaceutics: Jash Unadkat, PhD: jash@uw.edu
Dept. of Radiology: Steve Shoner PhD, scshoner@uw.edu